DNA computer (funded by NSF grant: SHF 1907824 ‘Development and manufacturing integrated DNA circuits’ )
Our goal is to construct a molecular scale processor based on DNA logic gates.
B-1. DNA four-way junction (4WJ) gates. We developed a series of 4WJ gates that can be used to build DNA circuits (Fig. 1). The outputs of the gates can be sensed by a molecular beacon (MB) probe. MB probe is a fluorophore- and a quencher-labeled DNA hairpin structure (Fig. 2A). Hybridization to a complementary DNA strand separates the fluorophore from the quencher, thus producing a fluorescent signal. The simplest YES gate (sensor) consits of strands A and B, each of which has a fragment complementary to an MB probe and a fragment complementary to an input oligonucleotide (Fig. 2B).34 In the absence of input, strands A and B do not bind the MB probe due to inability of unwinding the hairpin structure, while addition of an oliginucleotide input I1 leads to the formation of a stable 4WJ structure. NOT gate consists of a dumbbell-shaped DNA strand and two MB-binding arms forming a 4WJ complex with the MB probe in the absence of input (Fig. 2C). Addition of I1 decomposes the 4WJ structure an realizes MB probe leading to a low signal (digital 0). Figure 2D demonstrates the design of 2iAND gate. It consists of strands ANDa and ANDb, which form a 4 WJ structure with the MB probe only in the presence of both I1 and I2 inputs. I1 along can’t bridge the ANDa and ANDb due to the stable stem structure of the ANDa strand. The correct digital performance of the gates was reported in a series of publications. It is important to note that the outputs of 4WJ gates are new ~16 nt DNA sequences made two MB binding arms. These sequences can be recognized by the MB probe, but can also serve as inputs for downstream logic gates, as demonstrated below.
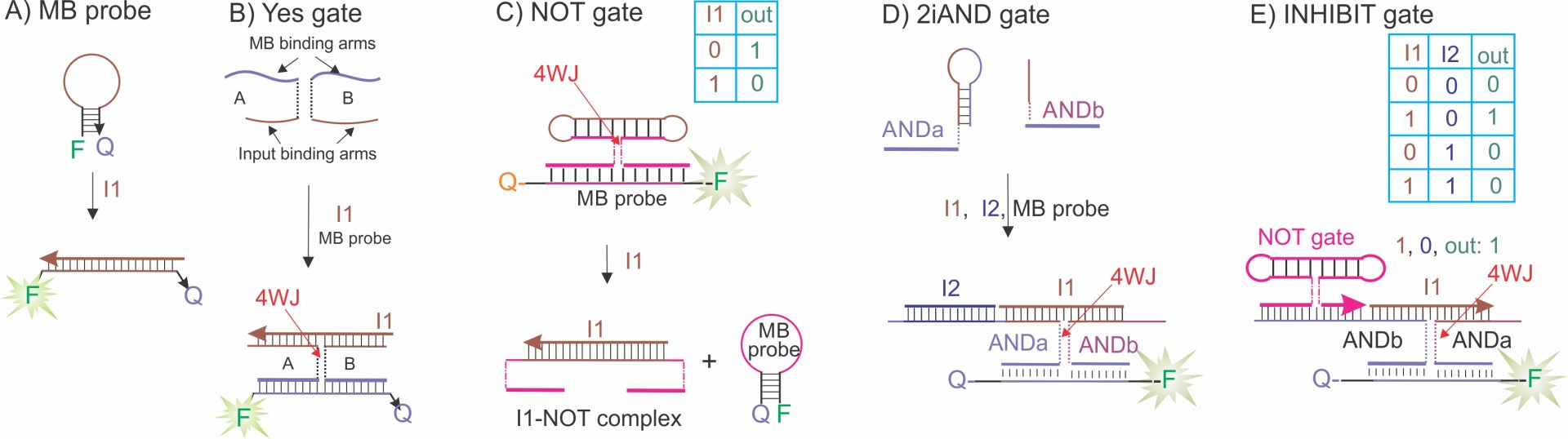
Figure 1. Design of 4WJ logic gates. A) Molecular beacon (MB) probe binds an oligonucleotide input and produces high fluorescence. B) 4WJ YES gate: strands A and B in the presence of input I1 hybridize to the MB probe and form a 4WJ complex. C) 4WJ NOT gate: a DNA strand (NOT) holds opened MB probe in the absence of an input. Addition of a DNA input decomposes the complex, thus releasing the MB probe. D) Two-input 4WJ AND gate (see the truth table in Fig. 1C): only in the presence of both I1 and I2, the highly fluorescent 4WJ structure is formed. E) INHIBIT gate is formed by having the output of NOT gate recognized as an input by the 2iAND gate.
2. Creating DNA circuits: integration of individual DNA logic gates in networks of communicating gates. Conventional computers use logic gates communicating with each other by electronic inputs and outputs. The long chains of communicating gates (circuits) can perform very complex logic tasks. However, majority of DNA computational units developed so far are represented by either individual logic gates or explore only several layers of integration. The problems of slow communication between DNA gates, as well as inter-gate crosstalk, are the two major factors that imped DNA logic gate integration in circuits. For example, the five-layered circuits of seesaw gates demonstrated an appropriate Boolean response only in several hours after input addition.
The 4WJ gate design preserves input/output homogeneity (both are nucleic acid sequences), which is important for the integration of logic gates into circuits. For example, Figure 2E demonstrates integration of a NOT with an AND gate to produce INHIBIT logic. The NOT and AND strands form a fluorescent complex with the MB probe only in the presence of I1 input, while addition of I2 decomposes the NOT gates followed by the collapse of the entire structure into separate strands. We achieved a 3-layer logic gate integration by designing an XOR gate made of one OR, two AND and two NOT gates in solution. Even with this simple system, the accurate Boolean response required over 60 min, which would make the DNA circuit impractical. To facilitate inter-gate communication and reduce the undesirable crosstalk, we confined the gates in a nano-environment by attaching them to a DNA scaffold and positioning them in proper orientation as described below.
3. Localizing DNA gates in nano-environment for efficient inter-gate communication. In moderncomputers, electronic gates are localized and oriented to the downstream gates on the surface of a silicon chip. In this design, an output of one gate travels short distance to arrive directly to the next gate in the chain of communicating gates, which makes inter-gate communication efficient. DNA nanotechnology offers a unique tool for localizing molecular components with a subnanometer precision.38-44 We took advantage of this technology by localizing 4WJ on a DNA tile.45 Fig. 3A demonstrates the design of a NOR gate made of two NOT and one OR gates.
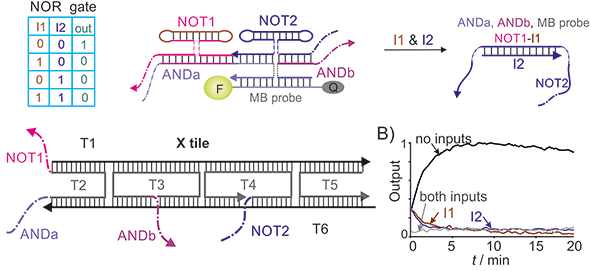
Figure 2. Integrated DNA circuits. Two-input 4WJ NOR gate integrated into a DNA tile.45 NOT1, NOT2, ANDa and ANDb strands were attached to triple crossover X tile at the indicated positions. In the absence of inputs, the terminal output fragments of both NOT gates are bound to the input-recognition fragments of the ANDa and ANDb, which enables formation of the high-signal (fluorescent) 4WJ NOR association. The addition of the I1 and/or I2 inputs, which are complementary to NOT1 and NOT2, respectively, results in dissociation of the 4WJ NOR complex.
When used tile-detached stands, NOT1, NOT2, ANDa and ANDb failed to communicate with each other. However, when attached to the DNA crossover tile (X-tile), the system of communicating strands expectedly produced high output only in the absence of both inputs (Fig. 3B). Importantly, the response of communicating gates was observed within the first 2 min after addition of the inputs, which was not the case for the gates communicating with each other in solution. Chatterjee et al. reported up to 8-layer chains of communicating logic gates on the surface of a DNA origami tile. Despite the gate communication required unwinding of relatively stable stem-loop DNA structures, the appropriate Boolean response of such circuits was achieved in minutes (up to 15-180 min for full response) after input addition. This data correlate with our observations and strongly suggests that localizing the gates on a DNA scaffold can enable building longer chains of communicating gates.
4. Powering DNA circuits. In semiconductor circuits, an electric input/output signal is pushed through the circuits by practically unlimited energy source – electric power supply. In DNA 4WJ gates, the high output complexes are maintained due to the energy released upon hybridization of a single oligonucleotide input, which is a limited source of energy. Extra energy is required for propagation of a signal through long chains of communicating gates, thus calling for a universal power supply.
DNA circuits can be powered using the principles of hybridization chain reaction. In this strategy, two partially complementary DNA hairpins remain unbound of each other (and therefore store energy) due to being caught in a kinetic trap in the form of intramolecular hairpins. An oligonucleotide input elevates the energetic barrier and triggers associations of the strands, which can now release the preserved energy. Seelig et al. powered DNA origami-bound circuits by a ‘fuel’ hairpin DNA present in solution as a buffer component. Each round of inter-gate communication, the fuel hairpin provided extra energy by hybridizing to the DNA-gate/input complex. This system is robust as it fuels each round of inter-gate communication. However, resetting of such system requires removing the fuel strand from the DNA circuits. Moreover, it exhibits relatively slow response due to the need for the toehold mediated hairpin hybridization. In this project, we propose to develop an alternative powering system based on the reactions catalyzed by Dz or RNases H enzyme with the goal to minimize fuel use and avoid stable dsDNA fragment to achieve high rates of inter-gate communication.
5 NAND logic gates. One obstacle in achieving efficient DNA gate integration and their fast response is the usage of DNA structures containing self-inhibited fragments (e.g. stem-loops or partial DNA duplexes) (Fig. 1-3). DNA inputs (and/or outputs of the upstream gates) must unwind the predesigned double stranded (ds)DNA fragments prior to triggering gate responses. For example, NOR gate of Fig. 2A responded to the inputs only after unwinding the ds dumbbell structures of NOT1 and NOT2 strands. The need to unwind stem-loops in DNA can significantly slow hybridization rates. In attempt to minimize the need for pre-designed dsDNA structures, we introduced NAND gates having minimal nucleotide involved in dsDNA fragments. NAND (negative AND, Fig. 4A) logic gate is functionally complete, since any Boolean function can be realized as a network of multiple communicating NAND gates. Therefore, NAND gate is an attractive module for building DNA circuits of arbitrary complexity. First, we designed a Dz-NAND gate attached to a DNA crossover tile (X-tile) (Fig. 3B).48 It is an association of four DNA strands: forward strand (FS), back strand (BS), and strands 1a and 1b (Figure 1C). The NAND function was fulfilled by the four functional fragments: BiDz-a, BiDz-b, Bridge Ī1 and Bridge Ī2, each of which was covalently linked to the DNA X-tile (shaded area in Fig. 3B). The two Bridge sequences were different to ensure recognition by different inputs, but both could hybridize to BiDz-a and BiDz-b, which would bring them together for F sub cleavage (digital 1). BiDz-a and BiDz-b hand only 5 nucleotides complementary to each of the bridges
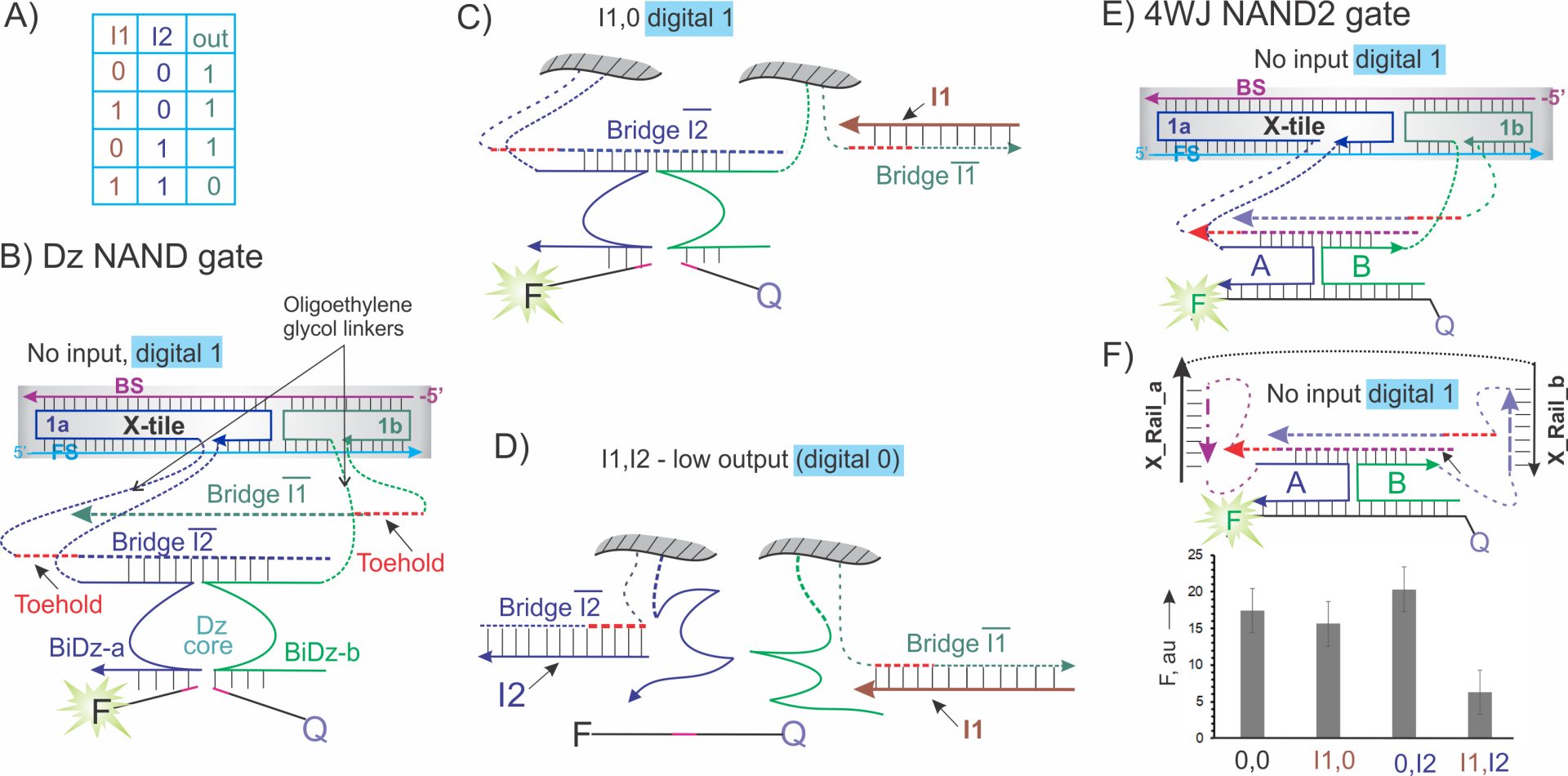
Figure 3. 4WJ NAND gates. A) Truth table. B) Dz NAND is an association of four oligonucleotide strands: front strand (FS), 1a, 1b, and the back strand (BS). Either Bridge Ī1 or Bridge Ī2 hybridize to BiDz-a and BiDz-b and form catalytically active Dz core, which produces high signal output. The Bridge fragments including toeholds (red dashed lines) are complementary to the corresponding inputs. Single-stranded DNA sequences are attached to the X-tile platform via flexible oligoethylene glycol linkers (green and blue dashed lines). C) High output (digital 1) is generated in the presence of one of the inputs, when either Bridge Ī1 or Bridge Ī2 remains to stabilize the catalytic core. D) In the presence of both inputs, Bridge Ī1 and Bridge Ī2 are both sequestered into duplex fragments and hence not available for stabilization of Dz core, thus leading to the catalytically inactive Dz (digital 0). E) 4WJ NAND gate functions as Dz NAND, but with the fluorescent signal being produced via hybridization with the MB probe rather than cleavage of a fluorogenic reporter. F) Two-input “railed tile” associated 4WJ NAND gate. Top: complex in the absence of inputs. Bottom: fluorescent response of the gate in the presence of different input combinations.
The Bridge fragments were fully complimentary to inputs I1 and I2. This made Bridge/input hybrids energetically more favorable than their complexes with BiDz-a and BiDz-b. In the absence of inputs, the NAND population should be roughly evenly split between the two associations, in which either Bridge a or Bridge b stabilizes the catalytic Dz core (Fig. 3B), since the predicted stabilities of the Bridge/BiDz arms hybrids for both Bridge sequences are similar. When only one input (I1 or I2) was present, it hybridized and inactivated its cognate Bridge, while the second bridging fragment still stabilizes the Dz catalytic core, thus ensuring high output (Fig 3C, in the presence of I1). At this stage, each input did not have to compete for binding the ‘bridge’, as there was always ~ 50% of Dz NAND population containing one or the other Bridge fragment in a single-stranded form. Hybridization of the 2nd input, however, proceeded via the DNA strand displacement, which is a competitive process. When both inputs are bound to their cognate Bridges, BiDz-a and BiDz-b turn into inactive split state (low fluorescence, digital 0, Fig. 3D). In our opinion, this was the most structurally relaxed system developed so far, which may contribute to shortening the response time and increasing the gate communication efficiency. Dz NAND gates demonstrated correct digital response within 5 min after input addition.48 Similar to Dz NAND gate, we designed a 4WJ NAND2 gate, which uses the MB probe for fluorescent signaling (Fig. 3E).48 Most recently, we adopted the 4WJ design for its integration into the ‘railed tile’ (Fig. 3F). The design is more flexible than for the X tile and helps do achieve cost-efficient integration of gates, as detailed below. The digital response of the gate was as expected (Fig. 3F, bottom).
6. Using our development in educational projects. We designed a construction kit for assembling DNA simple logic Boolean functions. It is assumed that the kit can be used by high school students either in class or at home. The design and performance of the logic gates is shown in Figure 6. The kit consists of a set of DNA strands that can make 2iAND and 2iINHIBIT functions controlled by the same I1 and I2 oligonucleotide inputs. The user is instructed to mix the indicated stands to assemble the two gates in separate sets of 4 tubes, which will be followed by adding all possible combinations of the same I1 and I2 inputs to the tubes. At input combinations corresponding to the low output, the gate strands remain dissociated in solution, thus producing no visible changes. Both gates form stable associations shown in Figure. 4A, D only in the presence of the input combinations that correspond to a high output. The associations possess a G-quadruplex structure, which can change the solution color or, initiate polymerization of acrylamide. In the last case, the liquid-to-gel transition output can be sensed by touch even by visually impaired people, which makes such detection format accessible to visually impaired people.57 The construction kit introduces the following concepts to the students: 1) principles of Boolean logic; 2) truth tables for AND and INHIBIT gates; 3) DNA self-assembly; 4) variations of computational outputs (e.g., electric, visible, etc.); 5) user-friendly outputs for visually impaired people. The data of this study is under preparation for publication in ACS Journal of Chemical Education. We plan to test the construction kit using a small group of high school students.
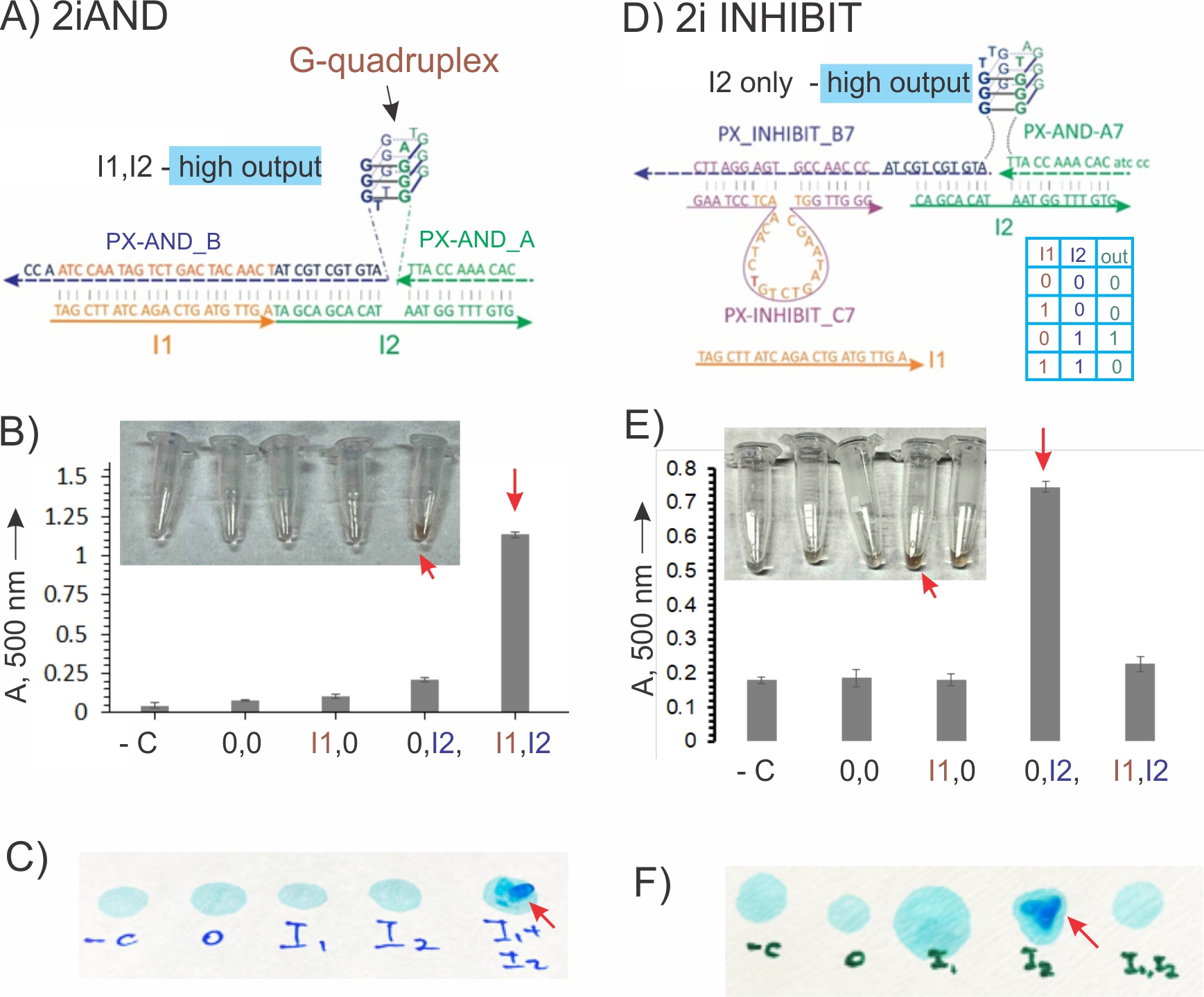
Figure 4. DNA logic gates with visual and tactile outputs. A-C) 2iAND gate. A) PX-AND_A and PX-AND_B in complex with I1 and I2 strands produce a G-quadruplex structure. B) Light absorbance at 500 nm and the photograph of the tubes with the samples containing 2iAND gate in the presence of different input combinations. C) The photograph of the filter paper with acrylamide solution containing 2iAND gate in the presence of different input combinations. D-F). 2iINHIBIT gate. D) The truth table and the G-quadruplex–containing structure formed in the presence of I2. E) Light absorbance at 500 nm and the photograph of the tubes of the 2iAND gate solutions in the presence of different input combinations. F) Photograph of the filter paper with acrylamide solution containing INHIBIT gate in the presence of different input combinations. Gel fragments are indicated by a red arrow. The solutions were stained with blue xylene cyanol dye for better visualization. The contrasts of the photographs were enhanced.