Prof. Sergey N. Krylov
Department of Chemistry, York University, Toronto, Ontario, Canada
Addressing Analytical Challenges of “DNA-Encoded Library” Technology
Abstract: Most therapeutic targets are regulatory or catalytic proteins, and modern drugs are developed to form stable complexes with them. Early-stage drug development aims to obtain and validate a large number of small-molecule hits capable of reversibly binding the target protein with acceptably low and known values of both the rate constant koff and the equilibrium constant Kd = koff/kon in reaction:
During later stages of drug development, validated hits are gradually reduced to pre-clinical leads, clinical candidates, and eventually, a drug.
Developing a single approved drug requires as many as 104 validated hits. Such a large number of them can only be reliably obtained from enormously-diverse combinatorial libraries of small molecules. The most diverse libraries, providing a means of reliable identification of hits, are DNA-encoded libraries (DELs), which are mixtures of up to 1012 compounds each with a DNA tag encoding its chemical structure.
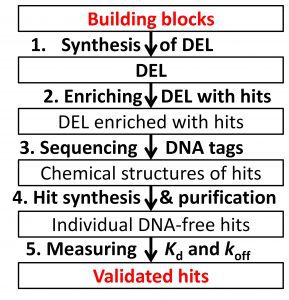
Validated hits are obtained from a DEL in five steps shown in the schematic: 1) synthesis of DEL from building blocks, 2) enriching hits in DEL, 3) sequencing the DNA tags to decode chemical structures of the hits, 4) synthesis/purification of DNA-free hits, and 5) measuring accurate Kd and koff for hit-target complexes.
Steps 1, 2, 4, and 5 currently rely on non-robust processes, which preclude streamlined production of validated hits even if these processes are put on robotic platforms. The non-robustness of these four steps creates costly delays in early-stage drug development. To address this challenge, we are developing four robust processes intended for eventual integration into streamlined manufacturing of validated hits. The 1st process is solid-phase non-aqueous synthesis of DELs from a great diversity of building blocks. The 2nd process is 1-round DEL enrichment with hits of desirably-low koff by capillary electrophoresis. The 3rd process is continuous-flow synthesis/ purification of DNA-free hits by integration of a continuous-flow microreactor with non-aqueous continuous-flow electrophoresis. The 4th process is accurate measurements of Kd and koff for hit–target complexes by combining kinetic chromatography with mass-spectrometry.
In this lecture, I will explain our progress in this ambitious research program. The focus will be made on our recent achievements including: 1-round selection of protein binders from DNA libraries [1–2], non-aqueous continuous-flow electrophoresis [3–5], and accurate measurements of Kd of protein–drug complexes [6–8].
- Le, A.T.H.; Krylova, S.M.; Kanoatov, M.; Desai, S.; Krylov, S.N. Ideal-filter capillary electrophoresis (IFCE) facilitates the one-step selection of aptamers. Chem. Int. Ed. 2019, 58, 2739–2743.
- Le, A.T.H; Krylova, S.M.; Krylov, S.N. Determination of the equilibrium constant and rate constant of protein–oligonucleotide complex dissociation under the conditions of ideal-filter capillary electrophoresis. Chem. 2019, 91, 8532–8539.
- Ivanov, N.A.; Lie, Y.; Kochmann, S.; Krylov, S.N. Non-aqueous continuous-flow electrophoresis (NACFE): separation complement for continuous-flow organic synthesis. Lab Chip2019, 19, 2156–2160.
- Ivanov, N.A.; Kochmann, S.; Krylov, S.N. Visualization of Streams of Small Organic Molecules in Continuous-Flow Electrophoresis. Chem. 2020, 92, 2907–2910.
- Kochmann, S.; Ivanov, N.A.; Lucas, K.S.; Krylov, S.N. Topino: A Graphical Tool for Quantitative Assessment of Molecular Stream Separations. Chem.2021, 93, 9980–9985.
- Sisavath, N.; Rukundo, J.L.; J.C.Y. LeBlanc; Galievsky, V.; Bao, J.; Kochmann, S.; Stasheuski, A.S.; Krylov, S.N. Transient incomplete separation facilitates finding accurate equilibrium dissociation constant of protein–small molecule complex. Chem. Int. Ed. 2019, 58, 6635–6639.
- Rukundo, J.-L.; Le Blanc, J.C.Y.; Kochmann, S.; Krylov, S.N. Assessing Accuracy of an Analytical Method in silico: Application to “Accurate Constant via Transient Incomplete Separation” (ACTIS). Chem. 2020, 92, 11973–11980.
- Rukundo, J.-L.; Kochmann, S.; Wang, T.Y.; Ivanov, N.A.; Le Blanc, J.C.Y.; Gorin, B.I. Krylov, S.N. Template Instrumentation for “Accurate Constant viaTransient Incomplete Separation” (ACTIS). Chem. 2021, 93, accepted.