Interests
Production of fuels and chemicals from sustainable sources, Synthesis of inorganic materials with an eye on applications in aerospace, optics and chemical feedstock. Examination of the kinetics and mechanism of solid-solid mechanochemical reactions. Development of Forensic analytical methods for the presumptive identification of substances of abuse.
Electron-Deficient Solids
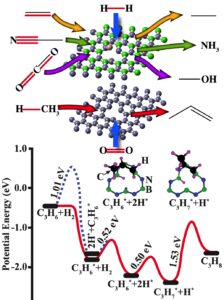
Our research explores how electron-deficient solids—particularly those based on Group 13 elements such as boron—can activate energy-relevant molecules through direct orbital interactions. These materials offer a metal-free platform for reactions traditionally catalyzed by transition metals, such as olefin and CO₂ hydrogenation, C–H activation, and methane coupling.
Catalysis at Defects and Boron Frameworks
Current work investigates the catalytic activity of structural defects in hexagonal boron nitride (h-BN) and related boron-rich frameworks. Defect sites in h-BN introduce localized electronic states that enable activation of C=C, C=O, and C–N bonds but also complicate spectroscopic analysis. To simplify and control these effects, we have developed all-boron frameworks that isolate and amplify active motifs such as nitrogen vacancies (VN). These materials exhibit promising activity for C–H bond activation and methane coupling, with ongoing studies quantifying steady-state reaction rates and activation energies.
Advancing Metal-Free Catalysis
Building on previous collaborations with CNRS Saint-Étienne (Dr. Laurène Tétard) and Brigham Young University (Dr. James Harper), we are integrating novel spectroscopic and solid-state NMR techniques to probe catalyst structure and reactivity at the atomic level. Parallel data-science tools developed at IDem Systems LLC are being applied to correlate structure–activity relationships.
Recent findings reveal that boron-rich solids outperform h-BN in oxidative dehydrogenation, leading to a UCF patent application (Blair) describing a boron framework catalyst that enables low-temperature (250–500 °C) methane coupling with stable production of C₃–C₄ hydrocarbons for over 17 hours.
Reaction Systems Under Study
Ongoing projects target two key transformations:
- Methane dehydroaromatization (MDA)
6 CH₄ → C₆H₆ + 9 H₂ (Currently funded by Compagnie de Saint-Gobain S.A.) - Reverse Haber–Bosch reaction
2 NH₃ → N₂ + 3 H₂ (Currently funded by NASA)
Integrating Fundamentals and Applications
Our research bridges fundamental and applied catalysis:
- Fundamental studies focus on synthesizing functionalized borophenes and correlating structure with activity using advanced solid-state NMR. The S-RESPDOR methodology pioneered by Dr. Harper enables direct distance measurements between ¹⁵N, ¹³C, and ¹¹B nuclei—providing unprecedented insights into adsorbate binding on catalytic surfaces.
- Applied investigations employ bench-scale reactors equipped with in situ Raman, IR, and mass-spectrometric probes to track spatial and temporal reaction dynamics. These data guide optimization of catalyst composition, reactor design, and overall energy efficiency.
Toward Scalable Boron-Based Catalysts
We are developing porous boron frameworks through topotactic removal of metals from borides, providing a scalable route to complex, high-surface-area materials. These boron-rich solids promise improved stability against oxygen and moisture, enhanced adsorption capacity, sulfur tolerance, and potential for sustainable large-scale chemical transformations
Photocatalysis and Solar-Driven Carbon Materials
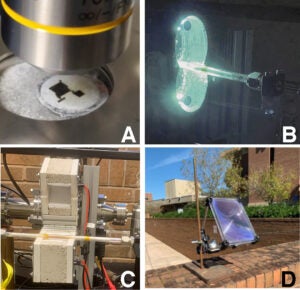
Photoreactor designs implemented A: Focused laser, B: LED lamps, C: HID lamps, and D: Solar. .
Our research investigates the photocatalytic potential of Group 13 elements, which span a remarkable electronic range from semiconductors (boron) to metals (tin). Modified boron and boron nitride structures synthesized in our lab have demonstrated promising photocatalytic activity, particularly for methane dehydrogenation and solar hydrogen generation.
To explore these reactions, we have developed a suite of custom photoreactors incorporating lasers, HID lamps, LEDs, and Fresnel lenses for focused solar illumination. These systems successfully enabled solar-driven hydrogen production from methane and propene, establishing proof-of-concept for sustainable hydrogen generation. Current work focuses on improving light penetration and thermal management, including designs with dual-elliptical reflectors, flow control, and solar tracking for outdoor testing.
Because hydrogen detection at low concentrations remains challenging, we are developing mass spectrometric techniques using a high-vacuum system and precision leak valves to quantify hydrogen evolution and establish detailed kinetic models of photocatalytic performance.
Enabling 3D Printing of Carbon Structures
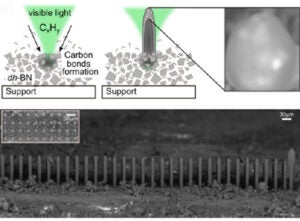
During photocatalytic hydrogen production, carbon by-products typically deactivate catalysts by covering active sites. Unexpectedly, Raman spectroscopy studies of active BN catalysts under hydrocarbon atmospheres revealed localized photocarbonization, producing microscale carbon features at temperatures below 200 °C.
In collaboration with Dr. Laurène Tétard (UCF/CNRS Saint-Étienne), we discovered that these conditions can be exploited to photo-pattern carbon microstructures without deactivating the catalyst—the active BN domains lift and integrate into the growing carbon. Building on this finding, we are adapting 3D printing technology to achieve controlled laser growth of carbon rods and frameworks.
Initial patterning on a confocal Raman microscope confirmed columnar growth, and subsequent tests on a custom Prusa-based 3D printer demonstrated feasibility, though mechanical limitations restricted precision. We are now developing a rigidized CoreXY platform in which only the laser head moves, enabling the controlled fabrication and study of complex carbon architectures formed via photocatalytic processes.
Inorganic Materials
Mechanochemical syntheses represent a scalable approach to the preparation of inorganic materials. We can perform reactions from 200 mg to 500 g.

Forensic Science
 Structural Analysis of Transition Metal Cluster Compounds for the Fluorescent Identification and Trace Detection of Substances of Abuse
Structural Analysis of Transition Metal Cluster Compounds for the Fluorescent Identification and Trace Detection of Substances of Abuse
Sensitive, rapid, high-sensitivity and low cost methods for detecting substances of abuse are being developed. This work focuses on studying the structure of fluorescent indicator complexes that enable the identification of substances of abuse with enhanced specificity. These fluorescent indicators are based on d10 metal complexes and allow greater detection sensitivity and flexibility. The indicators are shelf-stable, low-cost and the complexes formed can be stored for long periods of time without loss of fluorescence. The fluorescence observed in these complexes is due to the nature of the metal and metal-analyte bonds present in the complex. This is a well understood phenomena and will stand up to Daubert challenges.
With an understanding of the underlying molecular structure, computational methods can be utilized to predict any observed fluorescence and ultimately predict fluorescence for emerging substances of abuse such as fentanyl derivatives and xylazene.
X-ray Diffraction
Powder and single crystal X-ray diffraction are analytical methods that are very important to our group. Below is our recently reported structure of cubic CaSiN2.
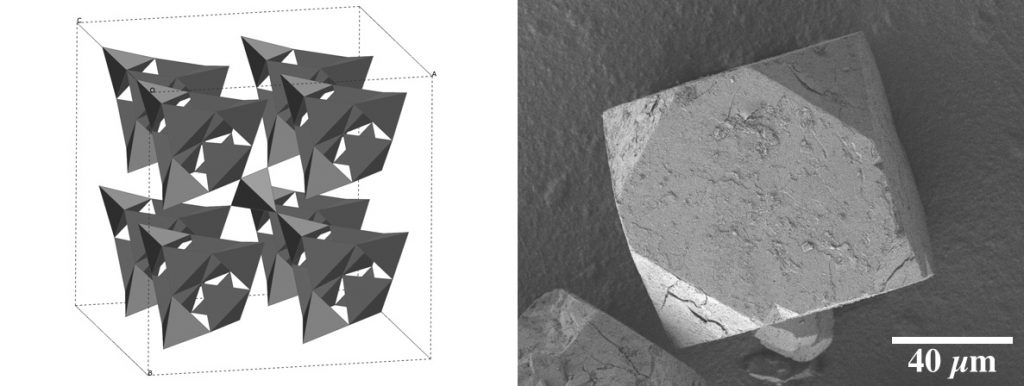
The identification of new compounds and phases of materials generated from solid-solid reactions are best identified by powder X-ray diffraction (XRD). We are also experienced in crystallite size and strain analysis of our materials through powder XRD. Our research focuses on new methods of the rapid synthesis of inorganic materials, examination of the mechanism of compound formation, and identification using X-ray diffraction.