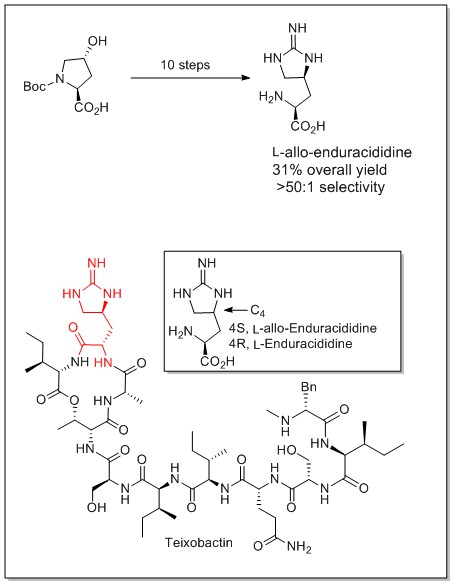
An asymmetric synthesis of carbocyclic spiroindoline by sequential Michael reaction and [3+2]-cycloaddition was achieved in our laboratories. This protocol demonstrates excellent enantio- and diastereoselectivity with broad functional group tolerance. A diverse range of spiroindolines were prepared by this approach and the products served as ideal substrates for C2 derivatization.
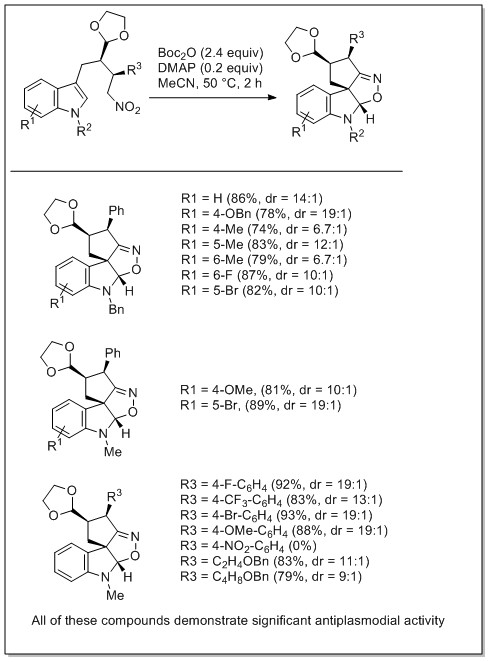
A highly stereoselective and scalable synthesis of l-allo-enduracididine from hydroxyproline derivative was recently disclosed by us. Pyrrolidine oxidation and reductive ring opening are the key steps in the synthesis. Compared to previously reported approaches, current route affords l-allo-enduracididine in 10 steps from Boc-trans-Hyp-OtBu in 31% overall yield with >50:1 diastereoselectivity.