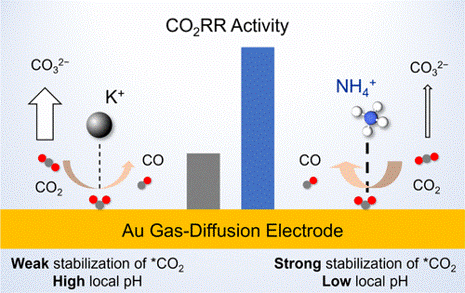
In collaboration with experimental groups at UCF, we have demonstrated that ammonium cations are more effective promoters of CO2 electroreduction (CO2RR) on Au in acidic media than Na+ and K+, achieving a 3-fold improvement in the CO2RR activity. Our Grand canonical Density Functional Theory (GC-DFT) simulations reveal that promotional effect of NH4+ cations is attributed to their enhanced electrostatic stabilization of CO2 adsorption, which is the rate-limiting step for the CO2RR on Au.
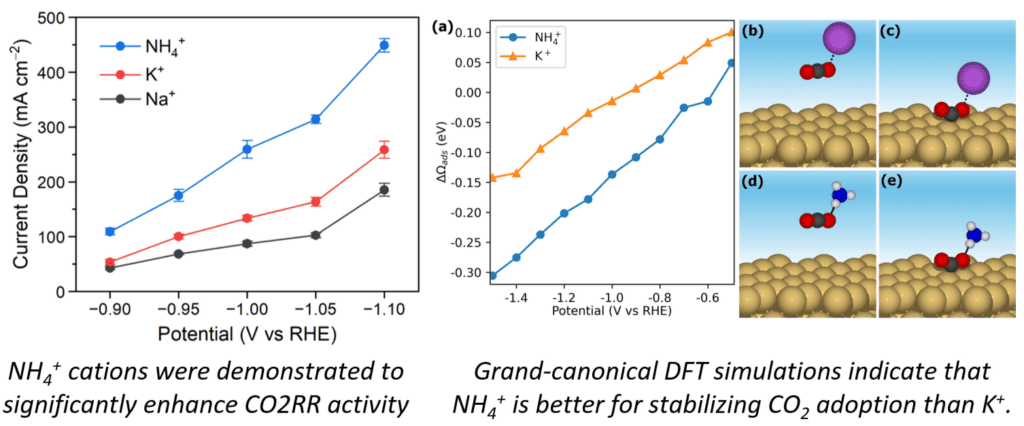
More details of this study can be found at: K. Shi, J. Janisch, Z. Ren, Z. Meng, D. Israel, D. Le, W.E. Kaden, T.S. Rahman, and X. Feng, “Ammonium Cation-Promoted CO2 Electroreduction on Au in Acidic Media,” Journal of the American Chemical Society 147, 23277-23285 (2025). http://doi.org/10.1021/jacs.5c08017