Interdisciplinary Research Leads to Promising Results for Future Alzheimer’s Disease Treatment
Researchers discover a more effective peptide-based treatment that targets aggregation of harmful amyloid beta proteins.
Written by: Emily Dougherty | Published: September 19, 2025
Interdisciplinary research between the College of Sciences (COS) and the Burnett School of Biomedical Sciences is looking into a more effective way to fight against the effects of Alzheimer’s Disease (AD) by homing in on protein amyloid beta, which worsens the condition over time for the affected.
The research team includes Professor of Physics Suren Tatulian from COS; Maria Zabala-Rodriguez, a graduate research assistant in the Burnett School of Biomedical Sciences; and Kenneth Teter, professor of medicine from the Burnett School of Biomedical Sciences. Their published research is supported by the Florida Department of Health, Ed and Ethel Moore Alzheimer’s Disease Research Program Grant 21A06.
According to the Alzheimer’s Organization, more than 7 million people in the United States are living with the disease. Tatulian says over time as the disease progresses, patients experience memory loss and the death of brain cells.

“Alzheimer’s disease is a neurodegenerative pathology characterized by neuronal death and brain atrophy,” Tatulian says. “Two proteins have been identified that contribute to the disease: amyloid beta and tau proteins, which are naturally occurring in the brain. However, uncontrolled, aberrant aggregation of these proteins causes neuronal dysfunction leading to Alzheimer’s.”
Tatulian explains how recently approved drugs by the U.S. Food and Drug Administration (FDA) focus on clearing amyloid beta aggregates, so he and his fellow researchers took an alternative approach that protects brain cells more precisely.
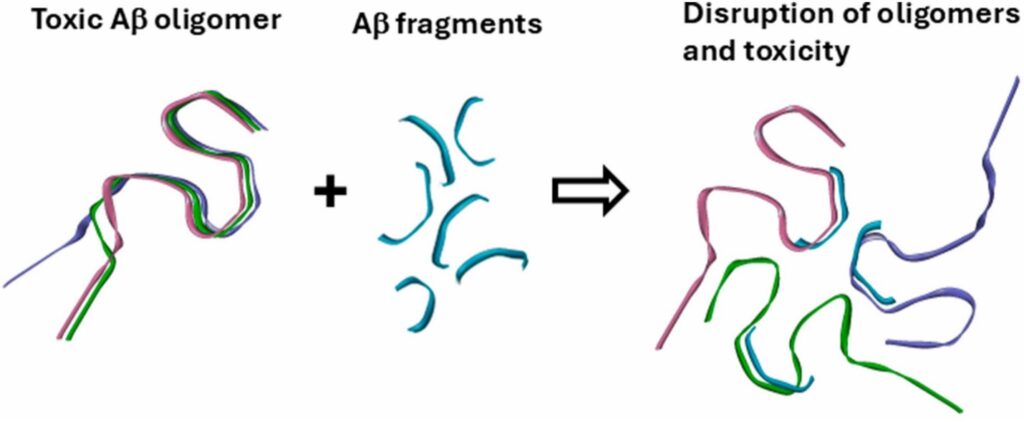
“Amyloid beta forms various types of aggregates, or clusters, mainly extracellular fibrillar plaques and intracellular oligomers,” Tatulian says. “The oligomers have been shown to exert the major cytotoxic effect. One of the established mechanisms is insertion of the oligomers into cellular membranes resulting in leakage of minerals and proteins culminating in neuronal death.”
He says fibrils, polymers containing hundreds to thousands of monomers, which can form as plaque on the brain, have been long associated with Alzheimer’s as well as with diseases that don’t cause neurotoxicity, so he and his team approached a different factor of the disease.
“Plaques were also found in the brains of healthy, Alzheimer’s-free individuals, implying they are not the toxic form of amyloid beta,” Tatulian says. “It turned out that the oligomers are the real toxic form that causes the damage through various routes including the membrane perforation mechanism. Hence, targeting the oligomers is potentially a more meaningful strategy of disease mitigation than targeting the fibrils.”
Small fragments of amyloid beta were tested by the research team to see if they could block the formation of the harmful oligomers earlier in disease progression.
“Protein aggregation starts with interaction between certain stretches,” Tatulian says. “Along the protein chain there are one or more sequences that are prone to aggregation (which can lead to the formation of fibrils). If you add to the protein solution a relatively short peptide corresponding to that sequence, it will interact with the respective stretch of the protein, block it and thereby prevent aggregation.”
To examine this approach, Tatulian and his fellow researchers tested seven synthetic peptides that covered the full length of the amyloid beta in hopes that it would prevent the formation of oligomers.
“Inhibition of fibrillogenesis is not the key—the peptide needs to inhibit the formation of the oligomers, not the fibrils, as the oligomers are much more toxic,” Tatulian says. “Indeed, in this work we identified three amyloid beta fragments that strongly inhibited amyloid beta toxicity against cultured cells.”
From this outcome, Tatulian says the study was able to identify why these amyloid beta fragments were cytoprotective.
“The requirements for their cytoprotective effect are the inability to form fibrils themselves, no effect on amyloid beta fibrillogenesis, suppression of amyloid beta oligomerization, and lack of toxicity,” Tatulian says.
He says this research puts a light on the potential for new types of drugs that use peptides, or small protein fragments, rather than antibodies for future Alzheimer’s Disease treatment.
“Although there are many peptide-based treatments for various diseases, currently there are no peptide drugs for Alzheimer’s,” Tatulian says. “On the other hand, peptides have advantages over antibodies, such as their small size which helps in crossing the blood-brain barrier and also allows intranasal administration (non-invasive treatment through the nasal passages).”
Tatulian says the research is still ongoing but is optimistic for the future it holds.
“Based on more research on peptides that inhibit amyloid beta toxicity, peptides will probably be recruited as effective Alzheimer’s drugs in the near future,” Tatulian says.
